本實驗室成功開發極具重要性之無殘留式醣蛋白專一性化學探針。並成功應用於醣蛋白晶片之研究。
此研究成果發表於聲譽卓著之美國化學會化學生物學期刊 (ACS Chem. Biol. 2014, 9, 390.)
http://pubs.acs.org/doi/abs/10.1021/cb400631w
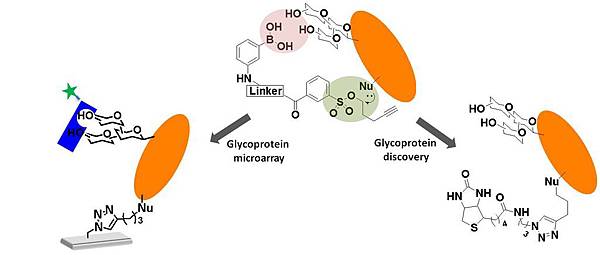
ABSTRACT: A new chemical method for the traceless labeling of glycoproteins with synthetic boronic acid (BA)-tosyl probes was successfully developed. The BA moiety acts as an affinity head to direct the formation of a cyclic boronate diester with the diol groups of glycans. Following this step, the electrophilic tosyl group is displaced by an SN2 reaction with a nucleophilic residue of the boronated glycoprotein, and finally, a reporter group is tagged onto the glycoprotein via an ether linkage. In the presence of polyols, a competition reaction recovers the native glycan of the tagged glycoprotein, conserving its biological significance. The BA-tosyl probes were used successfully for the specific labeling of glycosylated fetuins in a mixed protein pool and from crude Escherichia coli (E. coli) lysate. Further, a BA-tosyl–functionalized glass slide was used to fabricate glycoprotein microarrays with highly conserved glycans. By interacting with various lectins (carbohydrate-binding proteins), such as Concanavalin A (Con A) and wheat germ agglutinin (WGA), the types of carbohydrates and specific linkages of glycoproteins (α or β) could be systematically monitored. It is believed that the newly developed method will greatly accelerate the understanding of glycoproteins.
pochaou1002 發表在 痞客邦 留言(0) 人氣()
本實驗室於2014年之研究成果:A mild removal of Fmoc group using sodium azide
利用中性之溫和條件有效率進行Fmoc保護基團之移除。並成功應用於小分子及胜肽之合成
發表於:Amino Acids 2014, 46, 367.
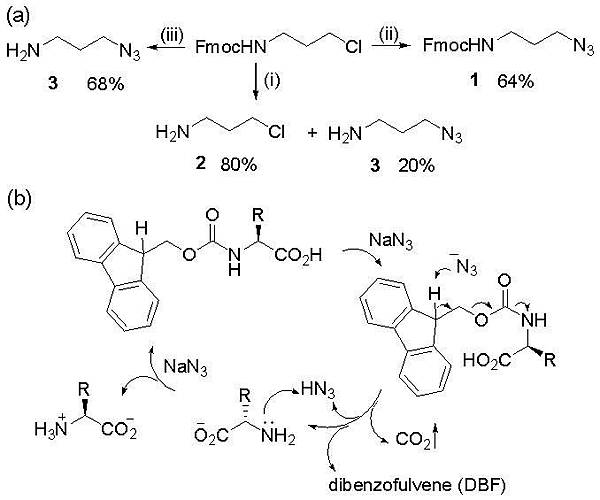
Abstract : A mild method for effectively removing the
fluorenylmethoxycarbonyl (Fmoc) group using sodium
azide was developed. Without base, sodium azide completely
deprotected Na-Fmoc-amino acids in hours. The
solvent-dependent conditions were carefully studied and
then optimized by screening different sodium azide
amounts and reaction temperatures. A variety of Fmocprotected
amino acids containing residues masked with
different protecting groups were efficiently and selectively
deprotected by the optimized reaction. Finally, a biologically
significant hexapeptide, angiotensin IV, was successfully
synthesized by solid phase peptide synthesis
using the developed sodium azide method for all Fmoc
removals. The base-free condition provides a complement
method for Fmoc deprotection in peptide chemistry and
modern organic synthesis.
pochaou1002 發表在 痞客邦 留言(0) 人氣()
本實驗室於2014年最新研究成果發表於美國化學會有機化學期刊 The Journal of Organic Chemistry
利用一價銅催化之Dihydropyrimidinone之合成及其在beta-amino acid衍申物之合成
後續之合成應用亦正在積極開發中!
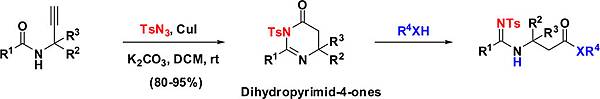
A copper(I)-catalyzed synthesis of substituted dihydropyrimidin-4-ones from propargyl amides via the formation
of ketenimine intermediate has been successfully developed; the synthesis afforded good isolated yields (80−95%). The mild
reaction conditions at room temperature allow the reaction to proceed to completion in a few hours without altering the
stereochemistry. Further, by involving a variety of reactive nucleophiles, the obtained substituted dihydropyrimidin-4-ones were
elegantly transformed into the corresponding β- and β3-amino acid analogues.
本研究工作發表於下列期刊:
J. Org. Chem. 2014, 79, 1254.
http://pubs.acs.org/doi/abs/10.1021/jo402670d
pochaou1002 發表在 痞客邦 留言(0) 人氣()
為了慰勞大家2013年一年的辛勞,歲末來一次墾丁行!!!
check in 中
 Sexy~
Sexy~
LPC group 卡丁車賽


LPC特戰隊!!



擺pose也只能趁現在了~

Double S girls

打到會變 笨!!

Ph. D

實驗室合照




LPC GO GO !
pochaou1002 發表在 痞客邦 留言(0) 人氣()
恭喜LPC group專題生:
楊晏靈、歐俊麟,推甄申請至台灣大學化學系
賴修平推薦申請至清華大學化學系
祝福他們的研究所研究能夠順利,也希望他們可以過得像現在一樣開心^^
pochaou1002 發表在 痞客邦 留言(1) 人氣()
實驗室各位同仁,由於老師本周末將進行晶片實驗,因此必須將實驗室原定本周六的seminar延後至下周六早上10:00。並於結束後,假
菩提廣場舉行每月的實驗室pizza會,希望與大家交換實驗上的收穫與面臨的困難。
pochaou1002 發表在 痞客邦 留言(0) 人氣()